IAA is affiliated with multiple research and development programs, allowing team members to work on cutting edge anesthesia research.
In our experimental randomized clinical trials, we formulate a novel anesthetic protocol.
After clearance from our institutional review board, we assign patients to treatment / non-treatment cohorts and use statistical methods to assess the efficacy of our intervention.
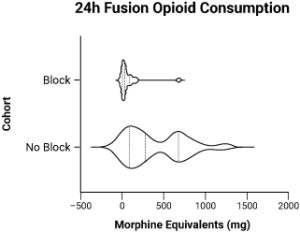
Example
A great example of this is our recent RCT of PENG nerve blocks for anterior total hip replacement [AAHKS 2021, T. Bowling et. al].
Requirements
Research trainees interested in randomized clinical trials should have a solid foundation in advanced statistics and study design.
Description
After developing a hypothesis, collaborating with our surgical colleagues, and acquiring clearance from our institutional review board, research trainees will work closely with senior anesthesia faculty in the process of formulating a feasible and generalizable clinical trial.
Research Experience Beneifts
IAA’s anesthesia research trainees learn:

Contact us for more information
Interested medical students and trainees are encouraged to email Dr. Saadat to discuss anesthesiology research opportunities with IAA. We hope you’ll consider joining our team.
Haleh Saadat, MD, FAAP
Director of Research, Fairfield Division
IAA Research & Development Department, Fairfield Division
haleh.saadat@iaapartners.com


Haleh Saadat, MD FAAP
Director of Clinical Research,
Fairfield Division
Bill Lahiff
Clinical Research Assistant
©2024 Integrated Anesthesiologists Association All Rights Reserved. PRIVACY POLICY